CYBERPSYCHOLOGY, BEHAVIOR, AND SOCIAL NETWORKING
Volume 16, Number 4, 2013
ª Mary Ann Liebert, Inc.
DOI: 10.1089/cyber.2013.1505
Heart Rate Variability and Biological Age: Implications for Health and Gaming
Carmen V. Russoniello, PhD,1 Yevgeniy N. Zhirnov, MD, PhD,2 Vadim I. Pougatchev, MD,2 and Evgueni N. Gribkov, PhD2
Abstract
Accurate and inexpensive psychophysiological equipment and software are needed to measure and monitor the autonomic nervous system for gaming and therapeutic purposes. The purpose of this study was to determine whether heart rate variability (HRV) derived from photoplethesmography (PPG) technology was predictive of autonomic nervous system (ANS) aging or biological age. Second, we sought to determine which HRV variable was most predictive of ANS change and aging. To test our hypotheses, we first conducted a criterion related validity study by comparing parameters of a 5 minute resting HRV test obtained from electrocardiography (ECG), the current “gold standard,” with PPG technologies, and found them to be significantly correlated (rq0.92) on all parameters during a resting state. PPG was strongly correlated to ECG on all HRV parameters during a paced six breaths per minute deep breathing test (rq0.98). Further analysis revealed that maximum variation of heart rate had the highest negative correlation (r = – 0.67) with age. We conclude that PPG is comparable to ECG in accuracy, and maximum variation of heart rate derived from a paced breathing test can be considered a marker of biological aging. Therapeutic interventions and games designed to reduce dysfunction in the ANS can now be developed using accurate physiological data.
Introduction
The human autonomic nervous system (ANS) affects the functioning of every organ system in the body. It is therefore not surprising that it is involved in virtually all physical diseases, whether infectious, inherited, neoplastic, or degenerative in nature.1 In addition, dysfunction of the ANS has been implicated in many mental disorders either as a cause, such as in posttraumatic stress disorder, or as a result of suffering from the illness, for example schizophrenia. The ANS adjusts or modifies many functions utilized in response to physical and psychological stress.2 If this system is weak or dysfunctional, problems can occur, including disease and death.3
Given these implications, the ability to evaluate the status of the human body quantitatively based upon ANS functional status becomes critical for a myriad of reasons. For example, data derived from these analyses can be used to aide in diagnosis of dysfunction and treatment outcome measurement. In addition, real time psychophysiological data can also provide individuals and healthcare practitioners with critical health information that can serve as objective feedback. Furthermore, games developers and programmers developing “health games” need methods to obtain accurate, physiological measurements in order to design stress reducing games appropriately.
Before a reliable measurement of ANS can be established, however, it is first necessary to separate the natural effects that occur in the ANS as a result of aging from other stressors on the system. The health game industry can use accurate and reliable information on the ANS to build games designed to reduce stress and increase ANS strength. Until recently, measurement of the ANS was a complex task involving a somewhat intrusive ECG in a clinical setting. Sensor technology that connects to a computer embedded in a blue tooth ear clip or part of an existing cell is now available. It is now possible to establish an accurate and reliable measurement of ANS change by developing standardized methods of measurement.
Measurement of the Autonomic Nervous System
Electrocardiography (ECG) is the well known method of measuring heart rhythm by recording electrical impulses generated by heart muscle,4 and is currently considered the ”gold” standard for HRV measurements.5 Heart rhythm obtained from ECG is currently considered the best way to detect normal sinus rhythm, as well as many types of cardiac arrhythmia. Information analyzed by ECG is also used to measure the functional status of the ANS.
Heart rate variability (HRV) is recognized as a robust method to evaluate the status of the ANS, which has regulatory control over the human body. HRV is considered a multidimensional measurement of effects of both sympathetic and parasympathetic activity, as well as some other physiological regulatory influences on the heart.6-8
Measuring ECG, however, is a cumbersome procedure requiring the placement of electrodes on the chest or limbs. It is typically performed in a physician’s office, clinic, or hospital, and is very expensive. These factors have prevented most clinicians from accessing this important information for assessment purposes and from using HRV to measure the efficacy of prescribed interventions.
For the past 50 years, researchers have been trying to develop alternatives to ECG that are easy to use, inexpensive, and can accurately capture the data necessary for HRV analysis. Photoplethysmography (PPG), or the measurement of pulse waves, is a recently developed alternative that has been reported to provide an accurate measure of heartbeat intervals used for HRV analysis.9 PPG was originally designed as a way to measure pulse waves generated by the heart pumping blood through blood vessels. It is based on the ability of hemoglobin to absorb light. Since the amount of hemoglobin passing through blood vessels changes due to the pulsatile nature of blood transportation, the amount of absorbed light also changes, generating regular pulse waves.9 PPG is measured with special optical sensors typically placed on the earlobes or fingers. Beat to beat changes in heartbeat intervals seen on an ECG are consistent with changes in time intervals between consecutive PPG pulse waves.
While research comparing HRV parameters derived from ECG and PPG recordings have been significantly correlated during 5-10 minute rest periods, no previous studies have compared HRV recordings of ECG and PPG during specific challenge tests like the six breaths per minute deep breathing test. The provocative deep breathing challenge is reflective of negative effects on ANS function, and therefore provides in- sight into the speed of ANS aging,10 and is considered as a standard method of assessing the body’s mechanisms of physiological adaptation.
Changes occurring in HRV with age were first reported in the 1980s.11-13 Researchers trying to understand differences in waveforms and their relationship to the ANS demonstrated that parasympathetic tonus significantly decreased with age.14 The usefulness of HRV analysis became immediately evident, and standards for HRV measurement were published in 1996. Computer based equipment capable of measuring beat to beat heartbeat intervals were designed, and the amount of studies in this area started growing exponentially.15-18 All of these studies supported earlier reports that indicated decreasing HRV is associated with the aging process.
It is well known that many other human physiological pa- rameters have strong correlations with age. Such correlations are found in various parameters of cardiovascular,19 endocrine,20 immune,21 and many other systems of the human organism.22 The human aging process causes irreversible morphological and physiological changes in human organs due to the gradual accumulation of damage to body cells, especially
in their genetic material-chromosomes. There is a substantial amount of published research data that demonstrates that distinct physiological changes occur in the ANS with age.23-26 Specificity was lacking, however, and this prompted researchers to consider which individual changes occurring in the autonomic function of healthy individuals was most predictive of aging. However, to determine this, normal physiological ranges needed to be established and correlated with age.
The purpose of this study therefore was to determine whether HRV data derived during the six breaths per minute deep breathing test could be correlated with age, and if so, whether there was a specific HRV parameter that could be used as a predictor of body aging. In order to answer this question, we conducted two studies. In the first study, we concurrently compared HRV parameters derived from ECG with the same parameters derived from PPG recorded at rest and during the deep breathing test. In the second study, we analyzed the HRV data collected in 5 minute resting and 1 minute deep breathing tests to determine which parameter had the highest correlation with age.
Methods
Participants
In the first study, there were 137 participants, ranging in age from 16 to 68 years. Of these, 69 were males with a mean age of 31.0 – 14.7, and 68 were female with a mean age of 33.0 – 12.4. Participants were excluded if they had any known health problems or had taken any medications in the 48 hours prior to testing. In the second study, there were 293 participants, ranging in age from 10 to 82 years. Of these, 121 were male with a mean age of 32.5 – 15.3, and 172 were female with a mean age of 38.6 – 15.7. Like the first study, participants did not have any known health problems and had not taken any medications for at least 48 hours prior to testing.
Equipment and data calculations
All participants were tested using the Biocom Heart Rhythm Scanner SE HRV analysis system (Biocom Technologies, Poulsbo, WA). ECG was recorded with USB ECG sensors at 256 samples per second rate. PPG was recorded with USB Pulse Wave Sen- sors at 240 samples per second rate. Both ECG and PPG signals were recorded in real time and stored for further analysis. After testing, each recording was verified with the software to make sure all abnormal heartbeat intervals were properly detected. All abnormal intervals were manually edited and replaced with values calculated using the linear interpolation method.
The following algorithm was used to derive heartbeat intervals from PPG pulse wave signal recordings. A raw PPG signal obtained from a USB pulse wave sensor was filtered using a digital band pass filter with a frequency band of 0.3-.0 Hz. This procedure ensured a very stable pulse wave free of baseline drift and with minimum waveform distortion. We then detected signal fragments with at least 10° positive slope. For each such fragment, minimum and maximum signal amplitudes, slope, and time between minimum and maximum amplitudes were calculated. For every 10 groups of these parameters, median values were found. Thirty percent of these median values were used as criteria levels for further detection of anacrotic limbs of pulse waves. Derivative values were calculated for every anacrotic limb of pulse waves, and then maximum values of derivatives for each such limb were found as cue points for calculating time in- tervals between consecutive pulse waves.
The following HRV tests were conducted in both studies: 5 minute resting HRV and 1 minute deep breathing test using a six breaths per minute pace. For the 5 minute resting HRV test, both time and frequency domain HRV parameters were calculated in accordance with the standards set forth by the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology. The following time domain parameters were calculated: mean heart rate value (HR), mean heartbeat interval value (RR), standard deviation from the mean RR value (SD), and root mean square of the standard deviation (RMS-SD). Before frequency domain parameters were calculated, the edited series of heartbeat intervals were resampled at a rate of two samples per second using a linear interpolation method. Fast Fourier transform method was used to calculate power spectra. The following frequency domain parameters were then calculated: total power (TP), very low frequency (VLF), low frequency (LF), high frequency (HF), normalized low frequency (LFnorm), normalized high frequency (HFnorm), and low frequency to high frequency ratio (LF/HF).
For the six breaths per minute deep breathing test, the following parameters were calculated: maximum ratio value between the longest heartbeat interval during expiration and
the shortest interval during inspiration (max E/I Ratio), mean ratio value between the longest heartbeat interval during expiration and the shortest interval during inspiration (mean E/I Ratio), maximum HR variation within one breathing cycle (max Variation HR), and mean HR variation among all breathing cycles (mean Variation HR).
HRV parameters calculated for each test were exported into a Microsoft Excel file. All data were grouped into one table according to the goals of this study for further statistical analysis using STATISTICA 6.0 software (StatSoft, Tulsa, OK).
Study protocol
During the tests, all participants remained in a slightly reclined (10%) sitting position. A standard reclined chair was used for testing. Dry ECG electrodes were placed on the left and right wrists of each participant, and were held secured under elastic wristbands. A PPG sensor was placed on the left index finger. ECG was not recorded in the second study. Before testing, all participants received the instruction to remain sitting relaxed and to have idle thoughts, limiting body movements. Each session started with the 5 minute resting HRV test. At the end of this test, participants were asked to remain sitting, and a new instruction to perform a 1 minute deep breathing test was given. During this test, participants must take six deep, even breaths synchronously with a visual breath pacer displayed on the screen. When the pacer moved up, the participant was instructed to take a deep breath in without forcing their breathing. When the pacer moved down, the participant was instructed to breathe out slowly. Compliance was closely monitored, as insufficient deep breathing or poor synchronization with the breath pacer can produce lower test results. The duration of each testing session including giving instructions was 7 minutes on average.
Results
Study 1
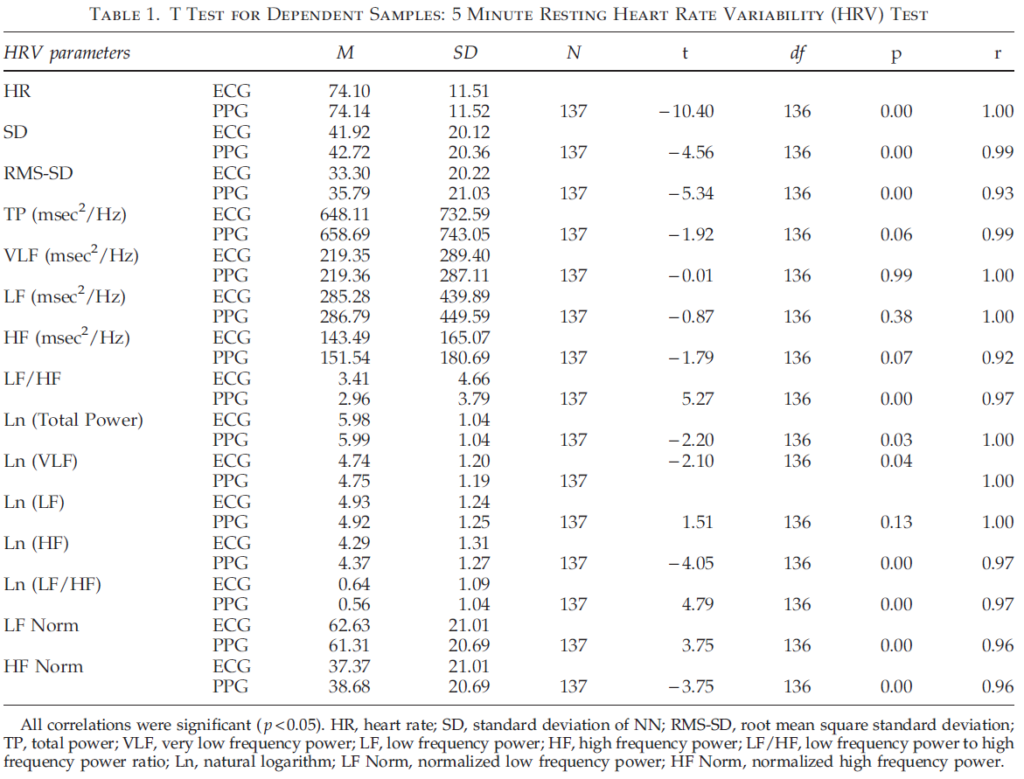
Table 1 shows the results of comparison of pairs of HRV parameters derived from ECG and PPG data. A t test for dependent samples was used to evaluate the difference between compared parameters. The results show significant differences between time domain parameters of the 5 minute resting HRV test obtained from ECG and PPG data. More thorough analysis of these data showed that, despite the fact that absolute values of HR, SD, and RMS-SD were very close, there was a shift toward larger values for parameters obtained from PPG data. Frequency domain parameters (total power, VLF, LF, and HF) did not demonstrate significant differences between the two samples, which can be explained by the large dispersion of these data. However, the frequency parameters’ natural logarithm values (Ln), LFnorm, and HFnorm had statistically significant differences with an exception of Ln (LF).
Table 1 also shows Pearson’s correlation coefficients between parameters calculated off heartbeat intervals derived from ECG and PPG. All pairs of parameters have a high correlation (rq0.92). Thus, our findings indicate HRV parameters obtained from PPG data provide an accurate assessment of HRV and support results published earlier.27,28
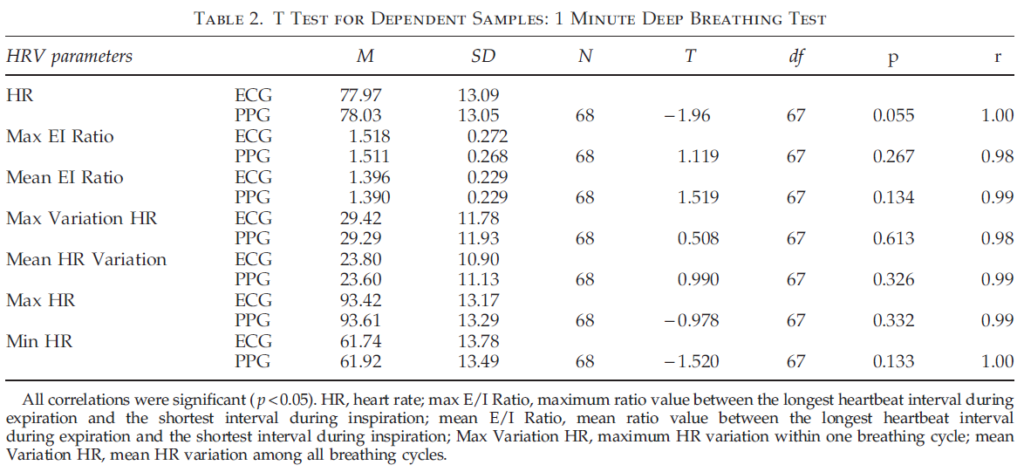
Table 2 illustrates the results of comparing ECG and PPG data during 1 minute deep breathing. The results indicate that all parameters did not statistically differ when t test for dependent samples were used. Moreover, the ECG and PPG parameters were highly correlated (rq0.98). Hence, the results of the first study indicate that HRV parameters measured with PPG technology give almost identical values with those measured with ECG, and confirm that PPG technology is a reliable and valid method of capturing and quantifying real time HRV data in studies utilizing both resting HRV and deep breathing tests.
Study 2
Table 3 contains Pearson’s correlation coefficients between parameters of the 5 minute resting HRV test and chronological age measured using PPG. All parameters except mean HR and LF/HF ratio show statistically significant negative correlations with age. The absence of a strong correlation between HR and age is consistent with earlier studies dem- onstrating that HR at rest does not depend on age.29
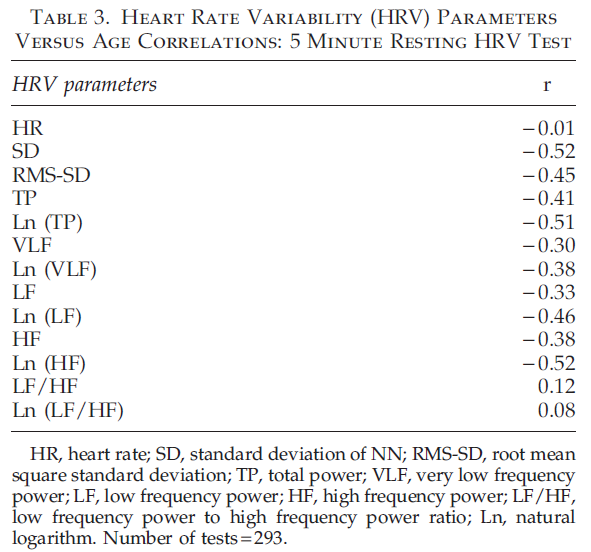
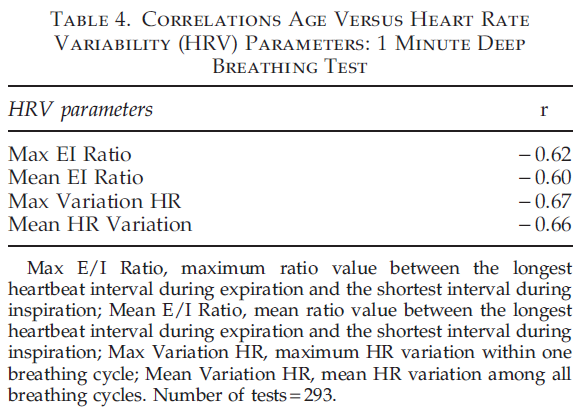
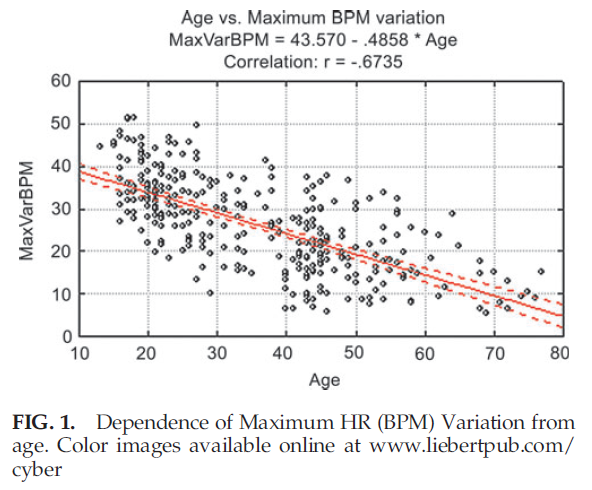
The absence of a strong correlation between LF/HF ratio and age can be explained because this ratio in healthy participants remains unchanged with age, as both LF and HF parameters equally change during a person’s lifetime. Correlations between HRV parameters on the 1 minute deep breathing test and age (Table 4) are significantly higher than parameters of the 5 minute resting HRV test, indicating the provocative test may be better able to detect the change in aging. The highest negative correlation during the 1 minute deep breathing test was found between maximum variation of HR and age. The graph illustrating age dependence of this parameter is shown in Figure 1.
Figure 1 demonstrates that the variance of maximum heart rate variability around its regression line is practically constant for all age groups. This variance can be explained by natural dispersion of this parameter, as well as by the possibility that calendar and biological age may be noticeably different in some participants whose data are presented on this graph.
Discussion
Study 1
Comparative analysis of HRV data obtained in this first study allowed us to conclude that PPG data may be used to calculate resting HRV parameters, and these data are com- parable to data obtained from traditional ECG, since PPG values of 5 minute resting HRV parameters derived from PPG were significantly different from respective parameters derived from ECG, which can be explained by the fact they were measured using two different technologies. Normal values for HRV parameters are dependent on age and gender, and therefore a normative database is needed to utilize PPG sensor technology properly for evaluative purposes.
Furthermore, this study was conducted on healthy participants. To establish wider application of PPG data for HRV analysis, it would be necessary to conduct similar comparative analyses of these two methods in patients with various health conditions affecting the autonomic function, such as diabetes, cardiovascular diseases, and so on.
The results of the six breaths per minute paced breathing test showed no significant differences, but there was a very high correlation between all HRV parameters when PPG and ECG recordings were compared. This fact supports the idea of wider use of the PPG measurement in challenge HRV tests. Such tests are more informative with regard to the autonomic function than the resting HRV test because they can help in assessing the adaptive capacity of the ANS. Overall, the findings of this first study support previous research on the usefulness of PPG data for conducting a full range of HRV-based assessments of the ANS in healthy participants. In the future, it would also be important to conduct similar comparative studies for other challenge HRV tests such as orthostatic, Valsalva, or handgrip test in healthy participants and patients with pathologies involving autonomic dysfunction.
Study 2
As noted above, there is a strong link between autonomic function and various health conditions reflected in HRV tests where a range of HRV parameters typically decrease with progression of a disease. The aging process may be considered as the sort of chronic condition that results in the slow deterioration of the body’s organs and functions, and the ANS is heavily involved in this process. This explains the strong negative correlation of HRV parameters with age demonstrated in a number of research studies. What was not known was which HRV parameter was most strongly related to these negative changes and age.
Deep breathing provokes the baroreflex-a key mechanism of the autonomic adaptive regulation-to operate at its capability level. It was shown that the baroreflex plays a key role in producing high amplitude modulation of the heart rate when breathing deeply at around six breaths per minute due to its intrinsic resonance response.30 However, its functional capacity degrades with age for various reasons, such as decreased elasticity of hardened arterial walls where baroreceptors are situated, decreased lung elasticity, decreased effectiveness of synaptic transmission (synaptic depression), and so on.31,32
As our results suggest, many of the parameters of the six breaths per minute deep breathing test have a high negative correlation with age in healthy participants, and could be considered as single markers of human body aging. One parameter-maximum variation of heart rate-had the highest correlation with age, and could serve as the marker of age when assessing ANS function. Further research is necessary to explore the applicability of this measure in people with various health conditions when accelerated aging is typical.
Conclusions
In this study, we demonstrated that maximum variation of heart rate provides an accurate assessment of current ANS function and, when compared to a normative database, can provide an individual with information on their biological age. We first tested the accuracy of PPG technology by conducting a criteria-related validity study with ECG. We found PPG to be accurate and reliable when recorded in a steady state. We then created a HRV normative database in healthy individuals using PPG technology. By comparing different HRV parameters in respect to a provocative test, we were able to determine that maximum variation of heart rate was the best predictor of ANS health.
These findings suggest that maximum variation of heart rate is a good method to determine the real time health of the ANS. By being able to monitor individual changes, healthcare practitioners can monitor the speed of ANS decline over time. If this aging process is faster than normal, then further tests could be conducted to determine the cause. If interventions such as medications or biofeedback are prescribed to affect the ANS positively, then changes in maximum variation of heart rate could be used as an outcome indicator. Individuals such as war fighters under extreme stress could be monitored for changes that could herald stress-related incidents such as combat fatigue or exhaustion. Sports medicine professionals and athletes needing to monitor the status of cardiovascular fitness could also use maximum variation of heart rate as an indicator of cardiovascular fitness. Finally, PPG technology was shown to be comparable to standard ECG measurement in this study. PPG technology is substantially cheaper than ECG, and does not require special equipment, disrobing, or expensive interpretation. PPG technology allows therapists access to a physiological marker for insight, measuring outcomes of interventions, biofeedback training, game development, and game control.
Author Disclosure Statement
No competing financial interests exist.
References
- Low P, Suarez G, Benarroch E. (1997) Clinical autonomic disorders: classification and clinical evluation in clinical auto- nomic disorders. 2nd ed. Philadelphia: Lippincott-Raven.
- American Heart Association. (2010) Autonomic nervous sys- tem. www.americanheart.org/presenter.jhtml?identifier = 4463 (accessed Aug. 10, 2010).
- Sapolsky R. (2004) Why zebras don’t get ulcers. New York: Herny Holt.
- Bickley LH. (1999). Bate’s guide to physical examination and history taking. 7th ed. Philadelphia: Lippincott Williams & Wilkins.
- Task Force of European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standard of measurement, physiological, and clinical use. European Heart Journal 1996; 17:354-81.
- Hayano JS. Accuracy of assessment of cardiac vagal tone by heart variability in normal subjects. American Journal of Cardiology 1991; 67:199-204.
- Moser MLS. Increase heart rate in depressed subjects in spite of unchanged autonomic balance? Journal of Affective Dis- orders 1998; 4:115-124.
- Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, et al. Origin of respiratory sinus arrhythmia in conscious humans. A important role for arterial carotid baroreceptors. Circulation 1997; 95:1813-1821.
- Cohen S, Kessler RC, Gordon LU. (1995). Strategies for measuring stress in studies of psychiatric and physical dis- orders. In Cohen S, Kessler RC, Gordon LU, eds. Measuring Stress: A guide for health and social scientists. NY: Oxford University Press.
- Shields RW. Heart rate variability with deep breathing as a clinical test of cardiovagal function. Cleveland Clinic Journal of Medicine 2009; 76:S37-S40.
- Pfeifer M, Weinberg C, Cook D, et al. Differential changes of autonomic nervous system function with age in man. American Journal of Medicine 1983; 75:249-58.
- O’Brien I, O’Hare P, Corrall R. Heart rate variability in healthy participants: effect of age and the derivation of normal ranges for tests of autonomic function. British Heart Journal 1986; 55:348-54.
- Jarisch JJ, Ferguson RP, Shannon JY, et al. Age-related dis- appearance of mayor-like heart rate waves. Cellular & Mo- lecular Life Sciences 1987; 1207-9.
- Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Heart & Circulatory Physiology 1987; 253:874-7.
- Umetahi K, Singer D, McCarty R, et al. 24-hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. Journal of the American College of Cardiology 1997; 31:593-601.
- Sinnreich R, Kark J, Friedlander Y, et al. Five minute re- cordings of heart rate variability for population studies: re- peatability and age-sex characteristics. Heart 1998; 80:156-62.
- Agelink M, Malessa R, Bruno B, et al. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clinical Autonomic Research 2001; 11:99-108.
- Bonnemeier H, Richardt G, Potratz J. Circadian profile of cardiac autonomic nervous modulation in healthy partici- pants: differing effects of aging and gender on heart rate variability. Journal of Cardiovascular Electrophysiology 2003; 14:791-9.
- Shimazu T, Tamura N, Shimazu K. Aging of the autonomic nervous system. Nippon Rinsho 2005; 63:973-7.
- Noth R, Mazzaferri E. Age and the endocrine system. Clinics in Geriatric Medicine 1985; 1:223-50.
- Edmond GA. Aging and the immune response: cellular and humoral aspects. Immunology 1987; 31:364.
- Cleophas T, Marum RV. Age-related decline in autonomic control of blood pressure: implications for the pharmaco- logical mangement of hypertension in the elderly. Drugs & Aging 2003; 20:313-9.
- Collins K, Exton-Smith A, James M, et al. Functional changes in autonomic nervous responses with ageing. Age and Ageing 1980; 9:17-24.
- Piha S. Cardiovascular autonomic reflex tests: normal re- sponses and age-related reference values. Clinical Physiol- ogy 1991; 11:277-90.
- Seals D, Taylor J, Ng A. et al. Exercise and aging: autonomic control of the circulation. Medicine and Science in Sports & Exercise 1994; 26:568-76.
- Galanter C, Wasserman G, Sloan R, et al. Changes in auto- nomic regulation with age: implications for psychophar- macologic treatments in children and adolescents. Journal of Child & Adolescent Psychopharamacology 1999; 9:257-65.
- Giardano N, Lehrer P, Edelberg R. Comparison of finger Ppethysmograph to ECG in the measurement of heart rate variability. Psychophysiology 2002; 39:246-53.
- Russoniello CV, Pougatchev V, Zirnov ME. A measurement of electroncardiography and photoplethesmography in ob- ese children. Applied Psycholophysiology & Biofeedback 2010; 35:257-9.
- Kostis J, Moreyra A, Amendo M, et al. The effect of age on heart rate in participants free of heart disease. Studies by ambulatory electrocardiography and maximal exercise stress test. Circulation 1982; 65:141-5.
- Vaschillo E, Lehrer P, Rishe N, et al. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Applied Psychophysiology & Biofeedback 2002; 27:1-27.
- Gribbin B, Pickering T, Slieight P, et al. (1971). Effect of age and high blood pressure on baroreflex sensitivity in man. Circulation 1971; 29:424-31.
- Gerritsen J, TenVoorde B, Dekker J, et al. Baroreflex sensi- tivity in the elderly: influnce of age, breathing and spectral methods. Clinical Science 2000; 99:371-81.
Address correspondence to:
Prof. Carmen V. Russoniello
Psychophysiology Lab and Biofeedback Clinic
Department of Recreation and Leisure Studies
College of Health and Human Performance
East Carolina University
Carol Belk Building Suite 2501
Greenville, NC 27858
E-mail: russonielloc@ecu.edu
